November 11, 2021
Life value
STELLAPHARM was born to care and protect patient’s health, to help enhancing their lives and living longer. Your health, for today and for future.
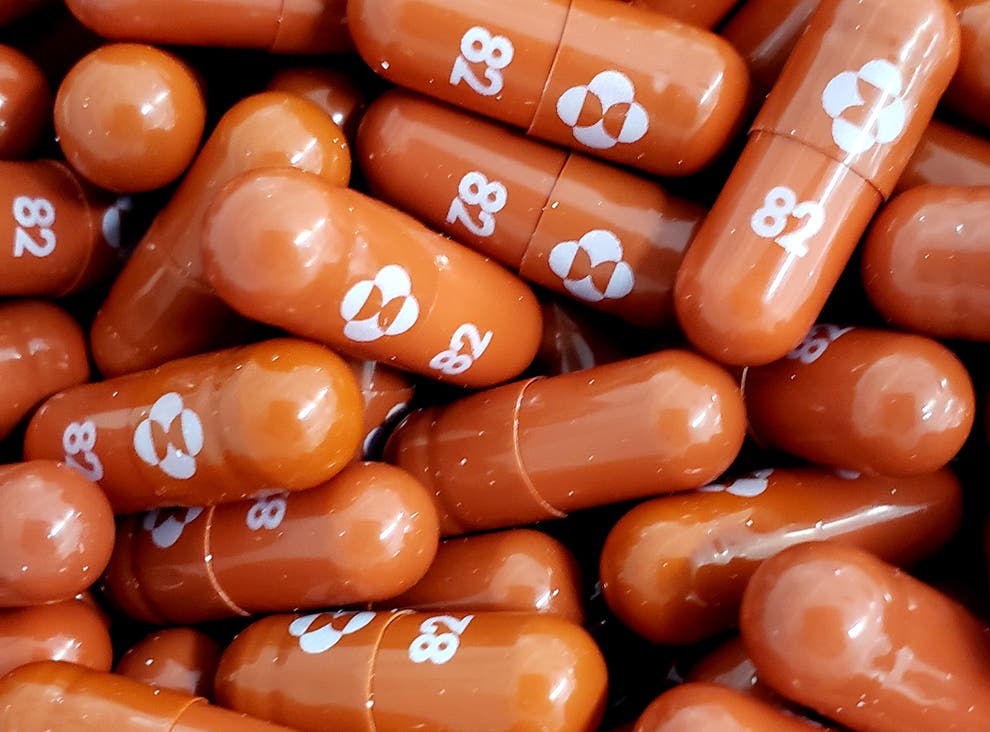
Molnupiravir can be taken as a pill instead of injection or intravenous administration.
Antiviral drug Molnupiravir, used for treating mild to moderate Covid-19 infections, is set to enter the Indian markets within days, an official confirmed on Wednesday.
Manufactured by US drug companies Merck, Sharp and Dohme, Molnupiravir is among the first proven drugs to effectively treat the viral contagion and was originally developed to treat flu.
The medicine could also bring down the risk of severe Covid-19 infections, two years after the coronavirus pandemic wrecked economies, torpedoed global trade chains, and brought international travel to a grinding halt.
It can be taken as a pill instead of injection or intravenous administration.
It could likely enter the Indian pharmaceutical markets “within days” after receiving Emergency Use Authorisation, Dr Ram Vishwakarma, chief of India’s Covid Strategy Group headed by the country’s top research body Council of Scientific and Industrial Research (CSIR), told Indian news channel NDTV.
“I think Molnupiravir will be already available to us. Five companies are sitting with the drug manufacturer… I think any day we will have approval of Molnupiravir,” Dr Vishwakarma said, confirming the drug’s introduction to India.
Molnupiravir will be priced much lower than what it is available in the US, pegged at US 700 dollars at present, the official said.
It could initially cost between Rs 2,000-4,000 (£20-40) for a single cycle of treatment and will slowly come down to Rs 500-1000 (£5-10), Dr Vishwakarma said.
“I think here when the government of India comes into play, they will buy in bulk from these companies and of course, they will have a dual pricing system and a staggered pricing system,” he said.
India, Mr Vishwakarma said, will also see another drug Paxlovid by Pfizer, which is a combination of two drugs, in some more time, the report added. The discussions are underway, Dr Vishwakarma said. Clinical trials by Pfizer showed that Paxlovid cut down the risk of hospitalisation or death by at least 89 per cent in vulnerable adults.
The third worst-hit country after the US and Brazil in terms of deaths and second in terms of total infections, India has seen 34,401,670 cases and 462,189 fatalities since the start of the pandemic.
Both the drugs are the “final nail in the coffin of the virus by science,” the chairman said.
India will not face the problem of availability of these drugs as the country will be needing billions of pills in bulk order depending on the course of the treatment, Dr Vishwakarma said.
This comes a week after Molnupiravir got clearance from the United Kingdom’s medicine regulator and is set to be administered twice a day to vulnerable patients diagnosed with the viral infection recently.
Health secretary Sajid Javed called the drug a “game-changer” for most frail and immunocompromised patients.
Source: Independent
Stellapharm is one of leading generics pharmaceutical companies and strong producer of anti-viral drugs in Vietnam. The company established in Vietnam in 2000; and focuses on both prescription drugs and non-prescription especially in cardiovascular diseases, antiviral drugs, anti-diabetics drugs, etc. and our products are now used by millions of patients in more than 50 countries worldwide.
The company is globally recognized for its quality through our facilities have been audited and approved by stringent authority like EMA, PMDA, Taiwan GMP, local WHO and others.
Additional information for this article: Stellapharm J.V. Co., Ltd. – Branch 1
A: 40 Tu Do Avenue, Vietnam – Singapore Industrial Park, An Phu Ward, Thuan An City, Binh Duong Province, Vietnam
T: +84 274 376 7470 | F: +84 274 376 7469 | E: info@stellapharm.com | W: www.stellapharm.com
On February 21, 2022, Stellapharm officially signed a contract with MPP (Medicines Patent Pool), a UNITED NATIONS-backed public health organization to become the only manufacturer in Vietnam allowed to produce a generic version of Pfizer’s oral COVID-19 treatment, nirmatrelvir, which will be co-packaged with ritonavir, under the voluntary licensing agreement between Pfizer and the MPP.
HÀ NỘI — The Drug Administration of Việt Nam under the Ministry of Health has issued the list of three COVID-19 treatment drugs produced in Việt Nam that contain the antiviral ingredient Molnupiravir, which have been granted certificates of registration for conditional use. The three products – Molravir 400mg produced by Boston Việt Nam Pharma (based in