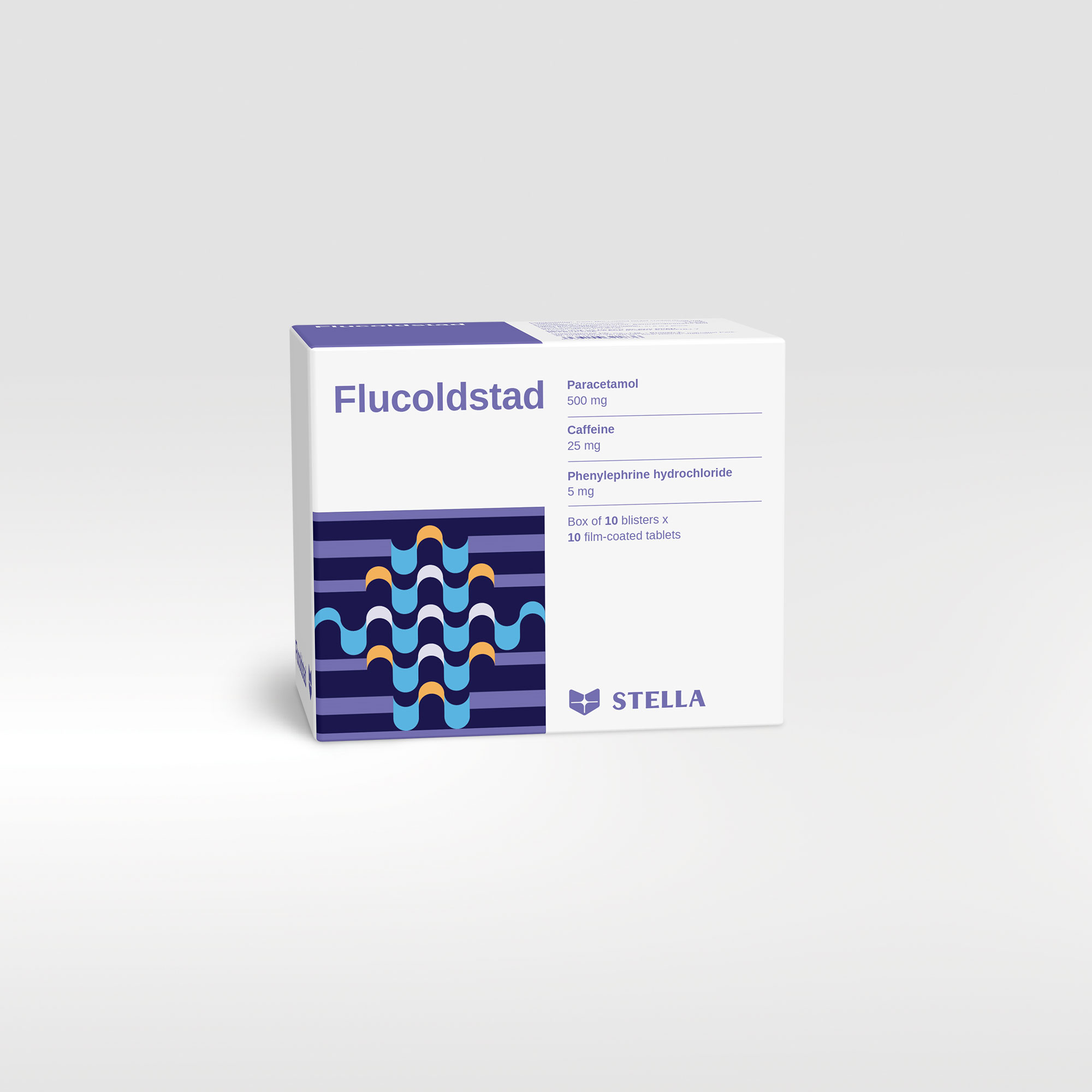
Flucoldstad OTC
Flucoldstad is fixed-combination of paracetamol, caffeine and phenylephrine that is indicated for the relief of sinus pain and the symptoms of colds and influenza, including fatigue and drowsiness.
| Pack size | Box of 100 tablets |
| Shelf-life | 24 months |
| Composition | Paracetamol, Caffeine, Phenylephrine hydrochloride |
| Dosage forms and strengths | Film-coated tablet: Paracetamol: 500 mg; Caffeine: 25 mg; Phenylephrine hydrochloride: 5 mg |