November 13, 2021
Life value
STELLAPHARM was born to care and protect patient’s health, to help enhancing their lives and living longer. Your health, for today and for future.
(Reuters) – The U.S. government will buy another $1 billion worth of the COVID-19 pill made by Merck & Co Inc and partner Ridgeback Biotherapeutics, the companies said on Tuesday.
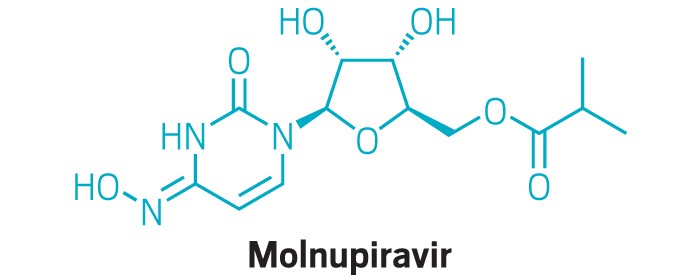
The government in June agreed to buy 1.7 million courses of molnupiravir for $1.2 billion and is now exercising options to buy 1.4 million more.
That brings the total secured courses to 3.1 million and worth $2.2 billion. Merck said the government has the right to buy 2 million more courses as part of the contract.
The drug has been closely watched since data last month showed that when given early in the illness it could halve the chances of dying or being hospitalized for those most at risk of developing severe COVID-19.
“Molnupiravir, if authorized, will be among the vaccines and medicines available to fight COVID-19 as part of our collective efforts to bring this pandemic to an end,” said Frank Clyburn, president of Merck’s human health business.
President Joe Biden said on Friday that the United States had also secured millions of doses of Pfizer Inc’s rival antiviral drug, which was shown to cut by 89% the chance of hospitalization or death for adults at risk of severe disease.
The Pfizer negotiations were for a deal similar to the one with Merck – 1.7 million courses of the treatment upfront with an additional option for 3.3 million, a senior U.S. health official said on Tuesday, confirming a New York Times report.
Pfizer Chief Executive Officer Alfred Bourla said on Friday that the company plans to sell its treatment for around the same price for high-income countries as Merck, at roughly $700 for a course of therapy.
Merck expects to produce 10 million courses of the treatment by the end of this year, with at least 20 million set to be manufactured in 2022.
Source: REUTERS
Stellapharm is one of leading generics pharmaceutical companies and strong producer of anti-viral drugs in Vietnam. The company established in Vietnam in 2000; and focuses on both prescription drugs and non-prescription especially in cardiovascular diseases, antiviral drugs, anti-diabetics drugs, etc. and our products are now used by millions of patients in more than 50 countries worldwide.
The company is globally recognized for its quality through our facilities have been audited and approved by stringent authority like EMA, PMDA, Taiwan GMP, local WHO and others.
Additional information for this article: Stellapharm J.V. Co., Ltd. – Branch 1
A: 40 Tu Do Avenue, Vietnam – Singapore Industrial Park, An Phu Ward, Thuan An City, Binh Duong Province, Vietnam
T: +84 274 376 7470 | F: +84 274 376 7469 | E: info@stellapharm.com | W: www.stellapharm.com
On February 21, 2022, Stellapharm officially signed a contract with MPP (Medicines Patent Pool), a UNITED NATIONS-backed public health organization to become the only manufacturer in Vietnam allowed to produce a generic version of Pfizer’s oral COVID-19 treatment, nirmatrelvir, which will be co-packaged with ritonavir, under the voluntary licensing agreement between Pfizer and the MPP.
HÀ NỘI — The Drug Administration of Việt Nam under the Ministry of Health has issued the list of three COVID-19 treatment drugs produced in Việt Nam that contain the antiviral ingredient Molnupiravir, which have been granted certificates of registration for conditional use. The three products – Molravir 400mg produced by Boston Việt Nam Pharma (based in