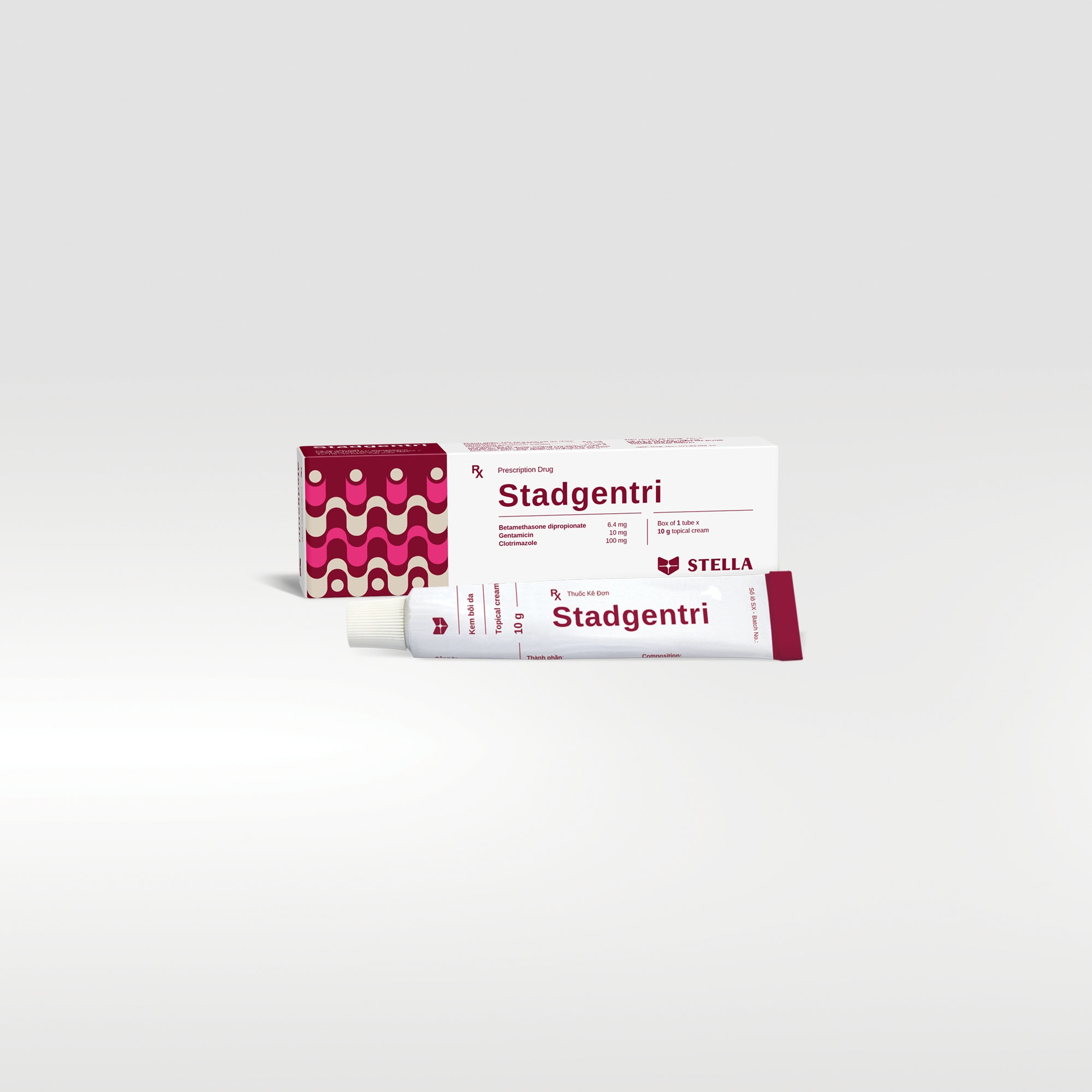
Stadgentri Rx
Stadgentri is a topical cream combining the anti-inflammatory, antipruritic actions of betamethasone with the antifungal activity of clotrimazole and the broad-spectrum antibiotic activity of gentamicin sulfate.
| Pack size | Box of 1 tube of 10 g, 20 g |
| Shelf-life | 24 months |
| Composition | Betamethasone Dipropionate, Gentamicin (sulfate), Clotrimazole |
| Dosage forms and strengths | Topical cream: Tube 10 g consists of Betamethasone dipropionate 6.4 mg, Gentamicin (sulfate) 10 mg, Clotrimazole 100 mg) Tube 20 g consists of Betamethasone dipropionate 12.8 mg, Gentamicin (sulfate) 20 mg, Clotrimazole 200 mg) |
Product code :